Asthma Management
A brief overview of asthma and its treatment.


Overview
This gallery features a brief overview of asthma and its management.


Asthma Management
Asthma has plagued humans (and animals as well) for well into recorded history. References to asthma date as far back as Hippocrates. As a major entity affecting health and well-being, a variety of remedies and treatments have been utilized throughout the ages.
In modern times, respiratory therapists have played a major role in the diagnosis, management, and education of patients with asthma.
Even in modern history, management strategies have undergone a huge transformation as new concepts, products, and research mean that a variety of treatment strategies undergo change.
A large driving force in the treatment strategy of asthma has been precipitated by the focus on evidence-based medicine and development of clinical practice guidelines.
Current research has resulted in a multitude of pharmacologic developments in the management of asthma. New medications are entering the marketplace at an ever increasing pace, and hopes for continued success in the management of asthma are on the horizon.
In modern times, respiratory therapists have played a major role in the diagnosis, management, and education of patients with asthma.
Even in modern history, management strategies have undergone a huge transformation as new concepts, products, and research mean that a variety of treatment strategies undergo change.
A large driving force in the treatment strategy of asthma has been precipitated by the focus on evidence-based medicine and development of clinical practice guidelines.
Current research has resulted in a multitude of pharmacologic developments in the management of asthma. New medications are entering the marketplace at an ever increasing pace, and hopes for continued success in the management of asthma are on the horizon.


Pre-1940
A variety of asthma treatments prior to 1940 are featured in this section of the gallery.


1778 Mudge's Inhaler
In 1778, John Mudge described the first commercial inhaler which was used in the treatment of asthma and other respiratory ailments.
Image from Mark Sanders


1860 Coffee Recommended
In 1860, Henry Hyde Slater recommended coffee to treat asthma. More recent analysis has shown that it contains the alkaloids caffeine, theophylline and aminophylline all of which are useful in the management of asthma.
Caffeine is a weak bronchodilator and reduces respiratory muscle fatigue.
Caffeine is a weak bronchodilator and reduces respiratory muscle fatigue.


Humphreys' "21"
Humphreys' "21" tablets, a homeopathic preparation for asthmatic paroxysms, contained Ipecac (Ipecacuanha), Indian Tobacco (Lobelia Inflata), and Arsenious Oxid (Arsenicum Album). The recommended dose was "."2 tablets for adults and 1 tablet for children every hour during paroxysm. "
In 1884, Dr. F. Humphreys, a professor of Homeopathic Medicine, authored a text that identified 35 specific compounds or mixtures for the treatment of 35 specific conditions. Asthma, "oppressed, difficult breathing, was treated with Humphreys' #21. Some of Humphreys' other homeopathic compounds are still marketed today.
In 1884, Dr. F. Humphreys, a professor of Homeopathic Medicine, authored a text that identified 35 specific compounds or mixtures for the treatment of 35 specific conditions. Asthma, "oppressed, difficult breathing, was treated with Humphreys' #21. Some of Humphreys' other homeopathic compounds are still marketed today.
Image from Felix Khusid


1889 Asthma Specific Inhaler
Dr. Nathan Tucker developed a nasal inhaler for his Asthma Specific Company.
Image from Gene Gantt
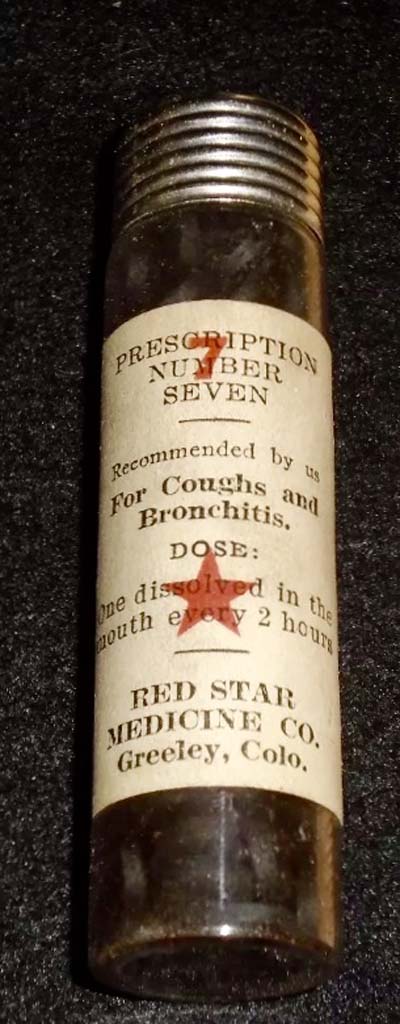

Red Star #7
The Red Star Medicine Company provided "treatments by number" for a variety of illness in the 1880s to early 1900s. The buyer told the store clerk his or her symptoms and the clerk selected the appropriate numbered treatments for the store display. This particular "prescription" --#7--- was used for relief of pulmonary symptoms.
Notice the product is "recommended by us".
Notice the product is "recommended by us".
Image from Felix Khusid


Dr. Tucker's Asthma Specific
Dr. Nathan Tucker's "Asthma Specific" was developed in 1889 and marketed into the 1930s. The aerosol delivery device was used to administer treatments for asthma which were found to contain between 1-3.5% cocaine.
Image from Felix Khusid


1890 Asthma Cigarettes
This 1890 ad is for Dr. Batty's "Asthma Cigarettes". Note that they were not recommended for children under 6 years of age.


Page's Inhalers
Page's Inhalers, medicated cigarettes were recommended "for the paroxysms of bronchial asthma". The "inhalers" contained stramonium leaves, chestnut leaves, tea leaves, gum benzoin, and kola nuts.
Users were instructed to "exhale the lungs of air, then after taking a mouthful of smoke, inhale the air into the lungs through the mouth allowing the smoke to go down with the air filling the lungs."
Users were warned to "discontinue use if rapid pulse or blurring of vision appears." The label also warns that the inhalers are "not to be taken by elderly people except on competent advice."
Although the product first appeared in the 1890s, ads promoting it as a treatment for asthma could be found into the 1950s.
Users were instructed to "exhale the lungs of air, then after taking a mouthful of smoke, inhale the air into the lungs through the mouth allowing the smoke to go down with the air filling the lungs."
Users were warned to "discontinue use if rapid pulse or blurring of vision appears." The label also warns that the inhalers are "not to be taken by elderly people except on competent advice."
Although the product first appeared in the 1890s, ads promoting it as a treatment for asthma could be found into the 1950s.
Image from Felix Khusid


Brater's Powder
The John K. Brater Company of Brooklyn, New York marketed Brater's Powder for "the temporary relief of paroxysms of asthma. The mixture contained stramonium, lobelia, and saltpetre.
Image from Felix Khusid


Regesan Relief for Asthma
Regesan Relief for Asthma was manufactured by Boots of Nottingham. The label recommends "burning a teaspoonful of the mixture four times a day and burning a tablespoonful of the mixture in the bedroom on retiring." The Regasan mixture was initially available circa 1912.
Images from Felix Khusid


1916 Vapo-Cresolene Ad
Asthma and other respiratory ailments were often treated with "drugless" therapies. This 1916 ad promoted vaporized Cresolene, a coal tar derivative "used while you sleep“. Purported to be a boon for asthma sufferers, it advertised that the “antiseptic vapor relieves bronchial complications of scarlet fever and measles” and was valuable in the “treatment of diphtheria as well as stopping the paroxysms of whooping cough and relieves the symptoms of spasmodic croup.” From The Vapo-Cresolene Co., New York City.


1922 Whiskey Prescription
During Prohibition, dispensing of alcohol was banned except for medical purposes. A prescription for alcohol ingestion was required.
A prescription for 1 tablespoon of whiskey prn prescribed for the treatment of asthma is shown.
A prescription for 1 tablespoon of whiskey prn prescribed for the treatment of asthma is shown.
Image from Felix Khusid


Powers Asthma Relief
E.C. Powers Company of Boston, Massachusetts began marketing "Powers Relief for Asthma Paroxysms" in the 1880s. The product contained stramonium alkaloid, mullein leaves, and saltpetre.
Image from Felix Khusid


Steam Tents
Asthma and other respiratory ailments were often treated by directing steam from a kettle into a tented enclosure. Herbs and fragrant oils and other home remedies were often added to the kettle.
Once hot showers became available in homes, the steam treatment was moved from the bedroom to the bathroom. In the decades that followed, many physicians continued to recommend placing children with asthma, croup, and other airway disorders in a steam-filled environment.
Once hot showers became available in homes, the steam treatment was moved from the bedroom to the bathroom. In the decades that followed, many physicians continued to recommend placing children with asthma, croup, and other airway disorders in a steam-filled environment.
Image from Tina Zerbo


Haywood's No. 401
Haywood's Improved Powder for the temporary relief of the paroxysms of asthma recommended that the user "ignite one-half to one teaspoonful of powder in a saucer and concentrate the fumes by placing over this a glass or paper funnel and inhale the fumes by deep breathing either through the nose or mouth. If necessary, repeat operation and confine vapor to your room and sleep in it." This product was available circa 1929.
Images from Felix Khusid
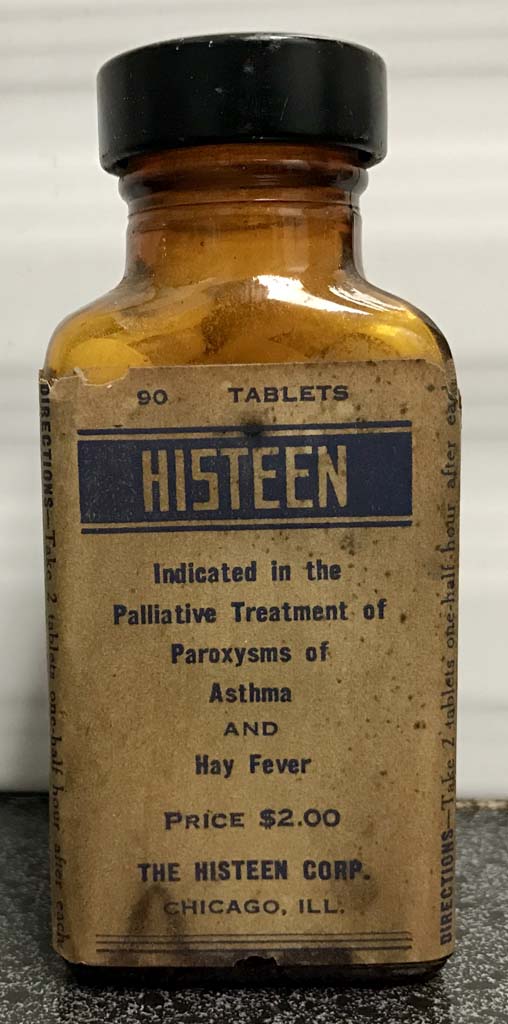
Histeen
Histeen pills were initially administered for the palliative treatment of paroxysms of asthma and hayfever but were banned in the 1930s when it was discovered the pills contained phenobarbitol.
Image from Felix Khusid


1930s Asthma Cigarettes
Asthma cigarettes and powders were popular in the 1920’s and 1930’s and many brands were available. They didn't contain tobacco, but rather crushed and dried herbs from the nightshade family of plants called solanaceae, which included datura strammonium (Thorn-apple plant), Atropa belladonna (Nightshade), Hyoscyamus niger (Henbane), Lobelia inflata (Puke weed) and similar plants. These plants contain the alkaloid, atropine, an anti-cholinergic. At the time, smoking wasn’t considered to be a hazard to health, but rather was actually considered to be beneficial.
After the introduction of rescue inhalers in the 1950’s, use of asthma cigarettes fell off.
After the introduction of rescue inhalers in the 1950’s, use of asthma cigarettes fell off.
Image from Felix Khusid


Himrod Medicinal Cigarettes
Himrod Medicinal Cigarettes (1930s-1940s) claimed to provide "temporary relief from the paroxysms of bronchial asthma." The cigarettes, manufactured in Brooklyn, New York by Himrod Mfg, Inc., contained sramonium, saltpetre, anise oil, and cedar oil.
It was recommended "to smoke one Himrod cigarette upon arising in the morning and one before retiring at night." In cases of mild paroxysms, it was recommended to smoke four cigarettes a day and in severe cases "to smoke more frequently."
The Himrod cigarettes are from the collection of Felix Khusid.
It was recommended "to smoke one Himrod cigarette upon arising in the morning and one before retiring at night." In cases of mild paroxysms, it was recommended to smoke four cigarettes a day and in severe cases "to smoke more frequently."
The Himrod cigarettes are from the collection of Felix Khusid.
Images from Felix Khusid
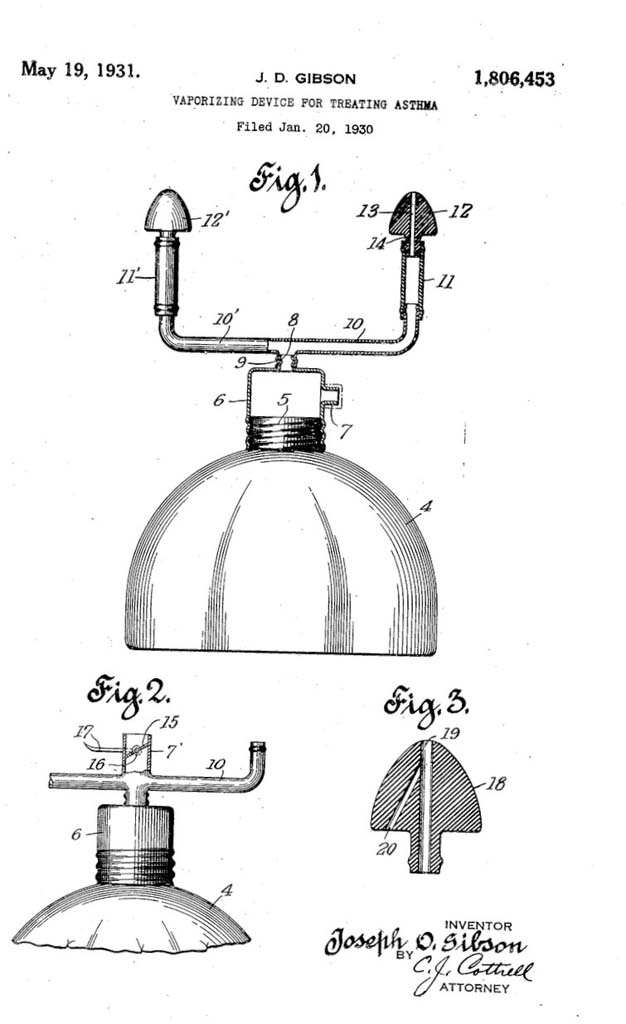
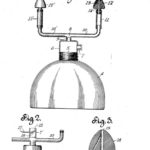
1931 Vaporizer for Asthma
Joseph D. Gibson filed an application for a patent for his "Vaporizing Device for Treating Asthma" in January 1930. The patent was awarded on May 19, 1931.
Image from the United States Patent Office

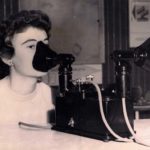
1934 Pneumostat
This device is known as the ‘Pneumostat’. An electric compressor was used to nebulize medications. Portable, electric nebulizers were introduced in the 1930s. The ’Pneumostat’ had an adjustable speed setting and weighed 9 pounds. It was made by Francis Riddell Limited in London and came with a carrying case.
Image from Steve and Mary DeGenaro


1936 Riddell Inhaler ad
A 1936 newspaper ad for a Riddell inhaler for home treatment of asthma is shown.


1936 Heliox Benefits Reported
Alvan L. Barach and Morris Eckman submitted an article for publication entitled "The Effects of Inhalation of Helium Mixed With Oxygen on the Mechanics of Respiration" in September 1935. The article was published on January 15, 1936 in the Journal of Clinical Investigation.
Since that time, heliox mixtures delivered in close fitting delivery devices have been used in the management of asthma exacerbations to decrease the work of breathing and enhance the delivery of oxygen to the gas exchange zone by aiding in laminar airflow throughout the tracheobronchial tree.
Since that time, heliox mixtures delivered in close fitting delivery devices have been used in the management of asthma exacerbations to decrease the work of breathing and enhance the delivery of oxygen to the gas exchange zone by aiding in laminar airflow throughout the tracheobronchial tree.
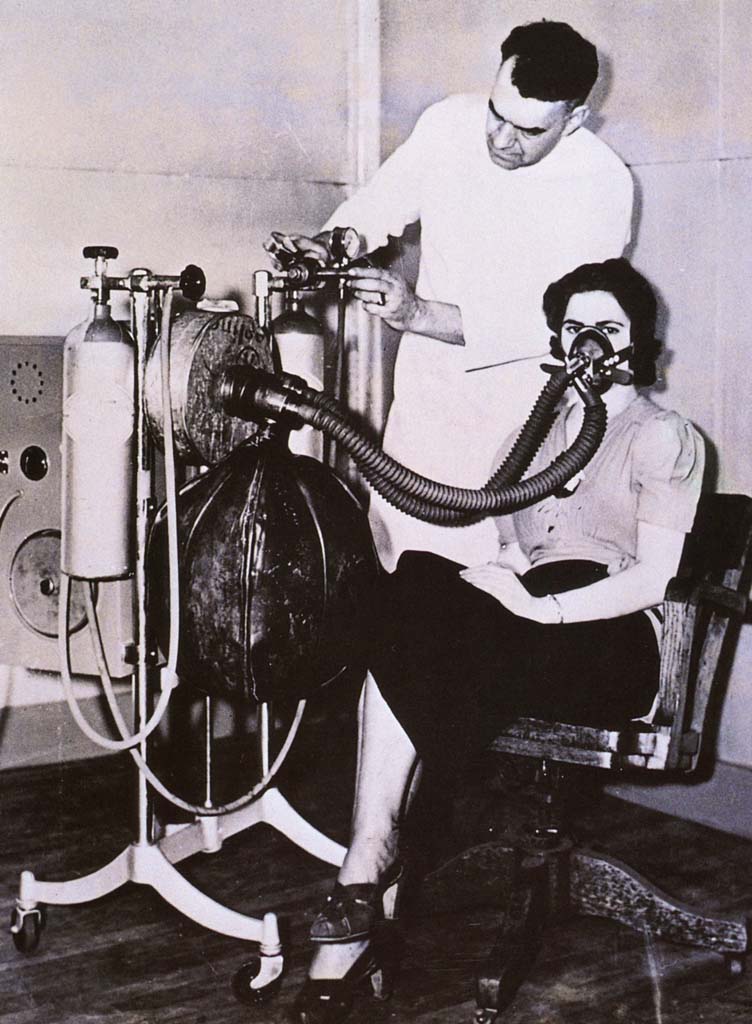
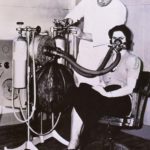
1938 Heliox Therapy
This 1938 photo taken at a Cleveland hospital shows the apparatus required to deliver helium and oxygen. The caption on the photo stated that "helium, an inert gas, when mixed with oxygen and found to be a more effective agent for 'artificial respiration' than pure oxygen."
Image from a collection from Aracely Bigelow


Kellogg's Asthma Relief
The side of the tin states: “For the relief of asthma and asthmatic hay fever. Place from one-half to a teaspoonful of the powder on a plate or a piece of metal, light it, then place an inverted funnel or lamp chimney over it an inhale the smoke freely, stirring occasionally until consumed. Repeat this as often as the spasms occur. We recommend that it be used on retiring as it impregnates the air with a soothing effect, thus allowing restful sleep."
Contains STRAMONIUM 31% LOBELIA 7 3/4%.
Manufactured by Northrop & Lyman Co., Toronto, Canada.
Contains STRAMONIUM 31% LOBELIA 7 3/4%.
Manufactured by Northrop & Lyman Co., Toronto, Canada.


1940s
Asthma treatments from the 1940s are featured in this section of the gallery.


1940s Asthmanefrin ad
A medical journal ad for Asthmanefrin is shown.
Image from Steve and Mary DeGenaro
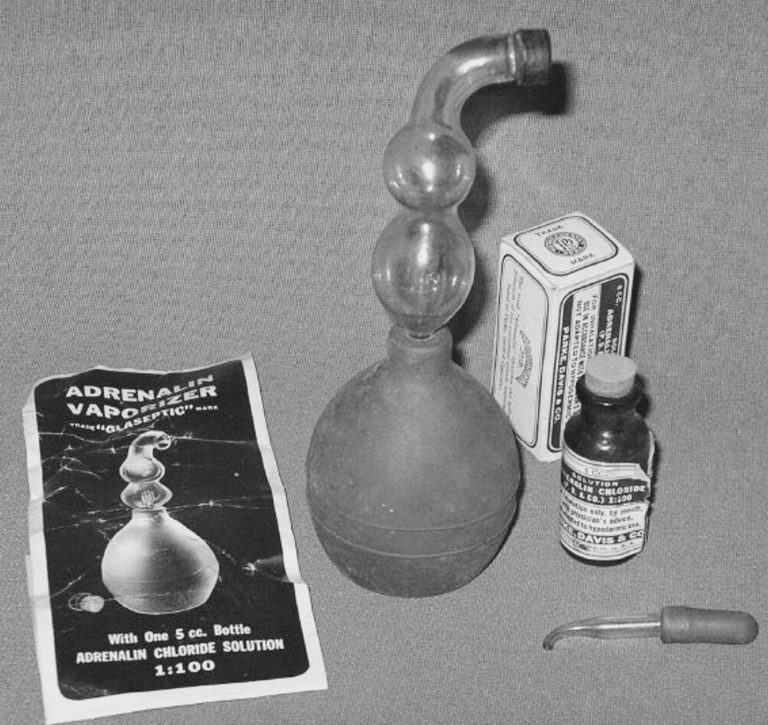
1947 Adrenalin introduced
Adrenalin chloride 1:100 was delivered via a glass nebulizer and bulb.
Image from Mark Sanders


Dr. Guild's Asthmatic Cigarettes
Dr. Guild's Green Mountain Asthmatic Cigarettes were prepared and distributed by the J.H. Guild Company of Rupert, Vermont. This label, copyrighted in 1947, is from the collection of Felix Khusid.
Image from Felix Khusid


Steam Therapy
Steam was often delivered for the home treatment of asthma congestion. The steam vaporizer pictured is from the 1940s.
Image from Felix Khusid


1950s
Asthma treatments from the 1950s are featured.


1954 Pocket Nebulizer
An ad for a pocket nebulizer from 1954 is pictured. It required 15-30 minutes of compression of the bulb to nebulize the average prescribed dose of meds.

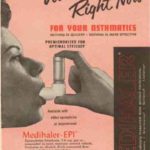
1955 MDIs Introduced
Metered-dose inhalers (MDIs) were first developed in 1955 by Riker Laboratories. The development of MDIs relied on the development of two different technologies—both relatively new at the time. That was the use of the chlorofluorocarbon (CFC) propellant and the metering valve which was originally intended for perfume. In 1956 Riker developed two MDI based products, the Medihaler-Epi containing epinephrine and the Medihaler-Iso containing Isoprenaline. As more beta-2 specific agonists have become available the use of these medications have largely fallen off.
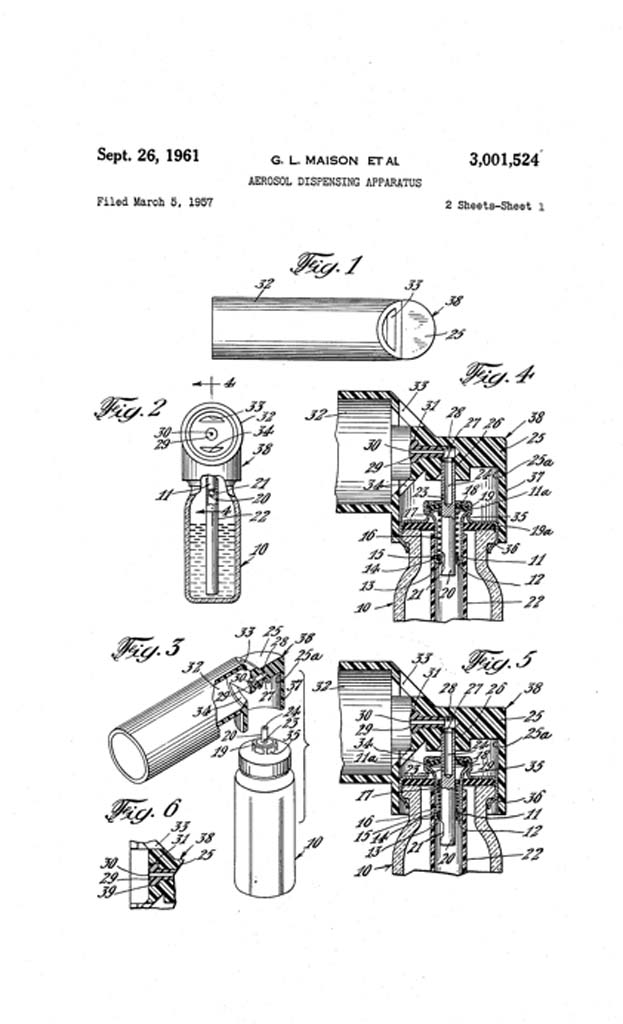
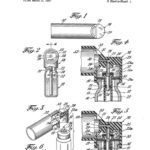
MDI Patent filed
In March 1957, G.L. Maison et al filed a patent application for "Aerosol Dispensing Apparatus". The patent was awarded on September 26, 1961.
Image from US Patent Office


Medihaler ad
Medihaler-Epi (0.5% epinephrine) and Medihaler-Iso (0.25% isoproterenol) were the first two MDIs offered in the 1950s.
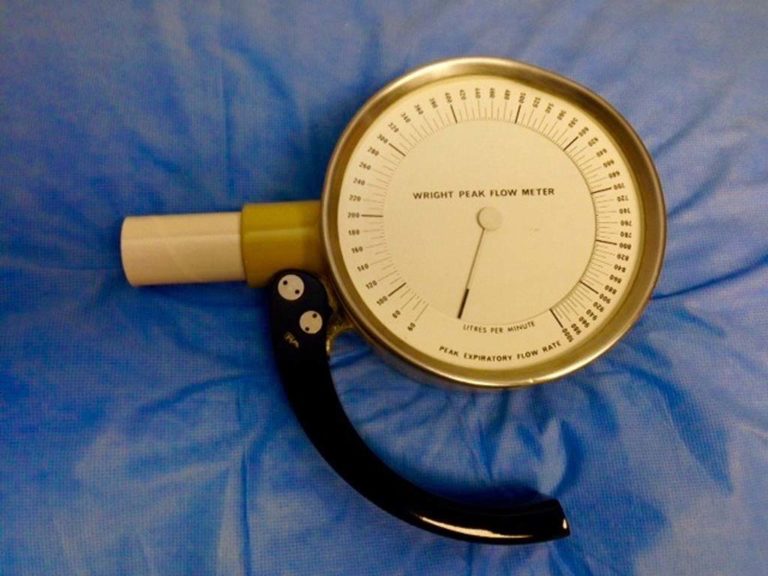

In 1956, Basil Martin Wright developed a monitoring device to measure peak expiratory flow. His device rapidly became the standard tool for monitoring the status of patients with asthma. The device pictured is from circa 1970 and used disposable mouthpieces. Single patient use devices are now readily available for home monitoring.
B. Martin Wright also invented the alcometer, commonly known as the Breathalyzer.
B. Martin Wright also invented the alcometer, commonly known as the Breathalyzer.
Image from Glenn Tammen


1960s
Asthma treatments from the 1960s are featured.


Monaghan M401 Peak Flow Meter
The Monaghan Peak Flow Meter was designed to monitor peak expiratory flows in pediatric and adult patients.
Image from James Sullivan


Isuprel Mistometer
Isoproterenol was prescribed for patients with asthma in an MDI known as a "Mistometer."
The drug affected the alpha, beta1 and beta2 receptor sites. Once drugs with minimal alpha and beta 1 stimulation were available in later years, the drug was rarely prescribed for asthma management.
Since asthma was thought to be caused by bronchspasm, the emphasis of treatment centered around bronchodilator therapy. It would take several decades before the medical community defined asthma as an inflammatory condition.
The drug affected the alpha, beta1 and beta2 receptor sites. Once drugs with minimal alpha and beta 1 stimulation were available in later years, the drug was rarely prescribed for asthma management.
Since asthma was thought to be caused by bronchspasm, the emphasis of treatment centered around bronchodilator therapy. It would take several decades before the medical community defined asthma as an inflammatory condition.
Image from Kerry George
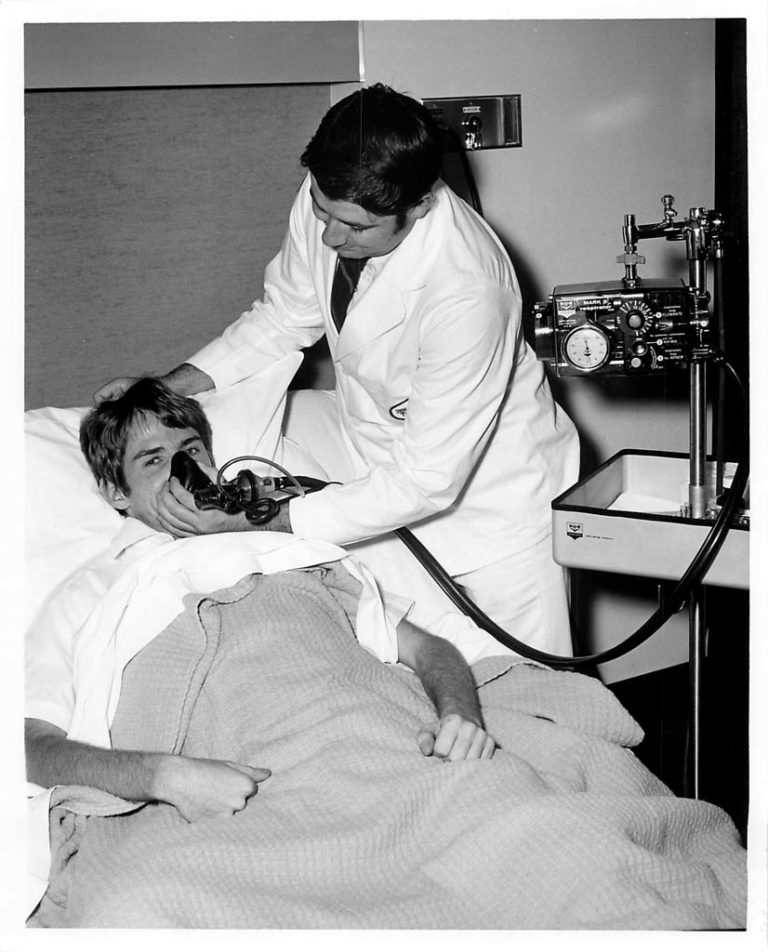
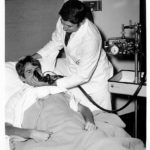
IPPB Treatments
Patients with asthma were often hospitalized during their exacerbations and treated with frequent IPPB treatments. Only a few bronchodilator short-acting agents were available to nebulize during the IPPB treatment, and often resulted in unwanted cardiovascular side effects.
Image from Aubrey Patterson


IPPB with Bronkosol 0.5 mL...
Bronkosol (isoetharine) emerged as the preferred bronchodilator to nebulize and administer via IPPB. It had some alpha and beta1 effects but to a lesser degree than Isuprel (isoproterenol), the other commonly prescribed bronchodilator for IPPB treatments.
Image from Kerry George


1968 Intal Spinhaler
Intal (cromolyn sodium) was introduced in the late 1960s. A capsule containing a dry powder of the agent, was inserted into the delivery device called the Spinhaler. The capsule was pierced and the powder was released and delivered to the patient on inspiration.
Image from Kerry George


1970s
Asthma treatments from the 1970s are featured.
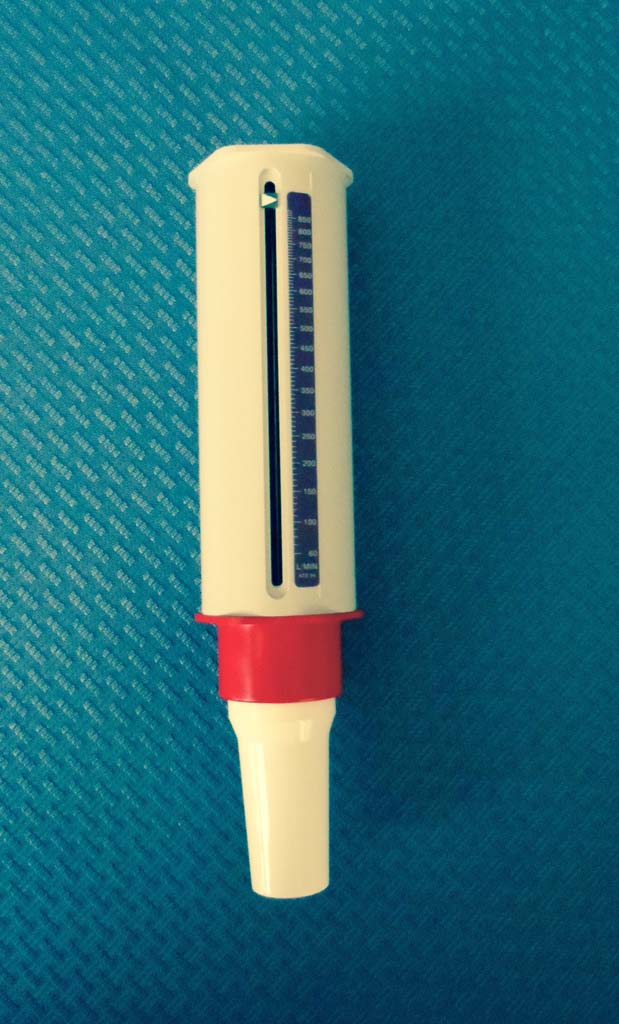
1970 Mini-Wright
In 1970, Basil Martin Wright introduced a portable peak flowmeter: the mini-Wight after originally designing a larger, version around 1950. The mini-Wright became standard equipment in medical offices, for pulmonary screening programs, and for home monitoring.
Image from Trudy Watson


1974 Sugarloaf Conference
In May of 1974, the National Heart and Lung Institute (NHLI)and the American Thoracic Society (ATS) sponsored a Conference on the Scientific Basis of Respiratory Therapy. The conference was held at the Sugarloaf Center in Philadelphia and became known as the Sugarloaf conference. The proceedings were published later that year in the American Review of Respiratory Disease. The conference addressed the scientific basis for respiratory therapy such as oxygen therapy, aerosol therapy, and intermittent positive pressure breathing. This conference was monumental in developing the scientific basis of respiratory therapy.
Conference proceedings addressed the effectiveness and efficacy of aerosolized medications.
Conference proceedings addressed the effectiveness and efficacy of aerosolized medications.


1975 - Bronchial Asthma
In 1975, CIBA published Clinical Symposia on Bronchial Asthma.
The cover features an illustration by Dr. Frank Netter.
Image from Trudy Watson
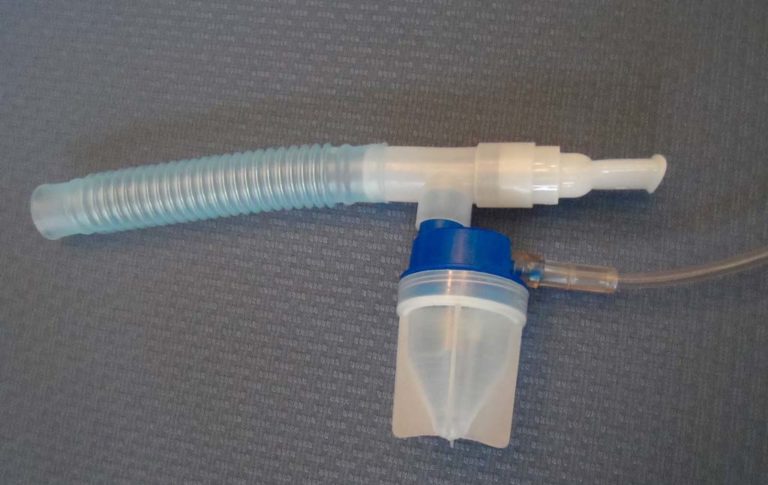
Nebulizer Usage Increased
The use of IPPB treatments to treat asthma declined following the publication of the Sugarloaf Conference in 1974. The use of aerosolized medications delivered via hand held nebulizers (also know as small volume nebulizers) increased rapidly.


Medi-Mist Compressor
As the use of IPPB declined following the Sugarloaf Conference, the use of small volume nebulizers increased. Portable, electrically powered compressors such as the one pictured were used to power the nebulizer.


1980s
Asthma treatments from the 1980s are featured.

1985 Mothers of Asthmatics (Now AAN)
In 1985, the Mothers of Asthmatics was established. It has now evolved into the Allergy & Asthma Network and continues to provide advocacy, education, outreach, and research programs.
Image from AAN website (www.allergyasthmanetwork.org)


1987 Montreal Protocol
The Montreal Protocol banned the use of chlorofluorocarbon (CFC) propellants as used in metered-dose inhalers. It was determined that CFCs were a threat to the ozone layer and thus impacted global warming. New propellants and alternative delivery devices such as dry-powdered inhalers make their way into the marketplace.
Image from the Environmental Protection Agency
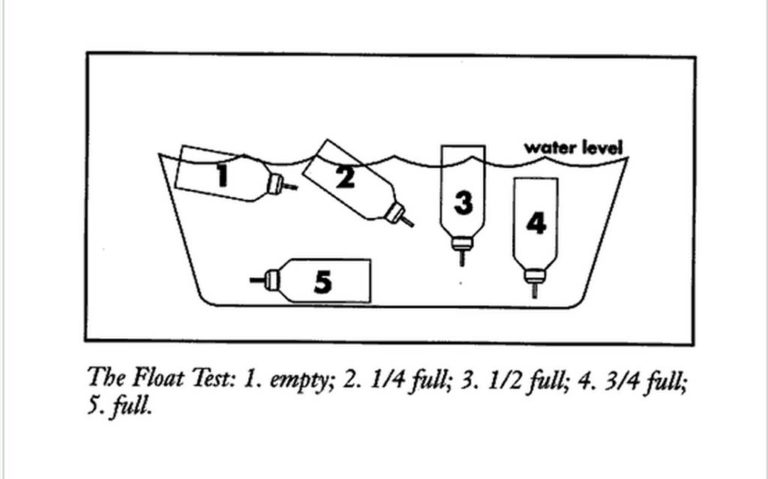
MDI Canister Float Test
Immersing an MDI's canister in water was once the standard method of determining the amount of medication in the canister. This practice was abandoned once CFC propellants were no longer used and dosage counters became available.

1989 NAEPP Initiated
The National Asthma Education and Prevention Program (NAEPP) was initiated in March 1989 to address the problem of asthma in the United States. The program is administered by the National Heart, Lung, Blood Institute to educate the patients, public, and health care professionals about asthma and its proper management
Image from NAEPP website (www.nhlbi.nih.gov/health-pro/resources/lung/naci/asthma-info/naepp.htm)


1990s
Asthma treatments from the 1990s are featured in this section of the gallery.


CFC-free Propellants
In this example from 1996, the dust cap for the actuator indicates the unit is CFC-free. The label on the canister indicates HFA is utilized as the propellant.
Image from Trudy Watson

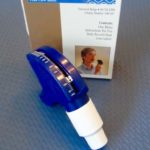
PocketPeak Peak Flow Meter
The PocketPeak flowmeter is one of many styles of compact peak flow meters available for easy assessment of peak flow.
Image from Trudy Watson


1991 Asthma Guidelines
The first Expert Panel Report "Guidelines for the Diagnosis and Management of Asthma" was published in 1991.
Image from Gayle Carr


Rescue vs. Controller Meds
For decades, the emphasis had been on the administration of bronchodilating agents. In the 1990s, the emphasis shifted to the use of anti-inflammatory agency to control persistent asthma in conjunction with long acting bronchodilators to control asthma symptoms (aka controllers). When acute symptoms flared, the administration of short acting bronchodilators was recommended (aka rescue meds).


ICS
After asthma was identified as an inflammatory disorder, inhaled corticosteroids (ICS) were recommended for persistent asthma. ICS may be offered alone or in combination with a bronchodilator in a delivery device.
Users must be reminded to rinse the oral cavity with water and expectorate after rinsing ("swish and spit") to reduce the incidence of oral candidiasis (thrush).
Users must be reminded to rinse the oral cavity with water and expectorate after rinsing ("swish and spit") to reduce the incidence of oral candidiasis (thrush).


Albuterol
Albuterol became the standard beta agonist used for "rescue inhalers", neb treatments, and continuous bronchodilator treatments.
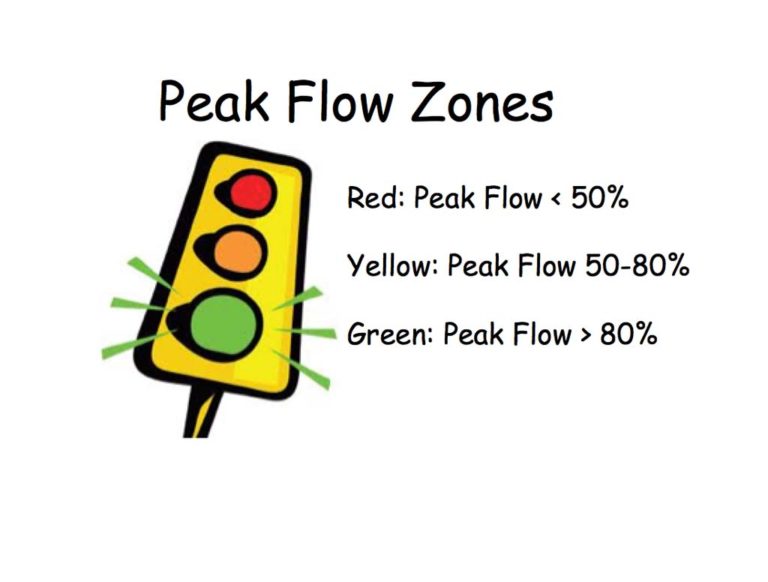

Peak Flow Zones
Understanding of the normal, warning, and critical peak flow values (often referred to as the green, yellow and red zones) is important for successful asthma management. Modification to medication dosage and frequency may be required when peak flow values change from normal values.
Image from Trudy Watson
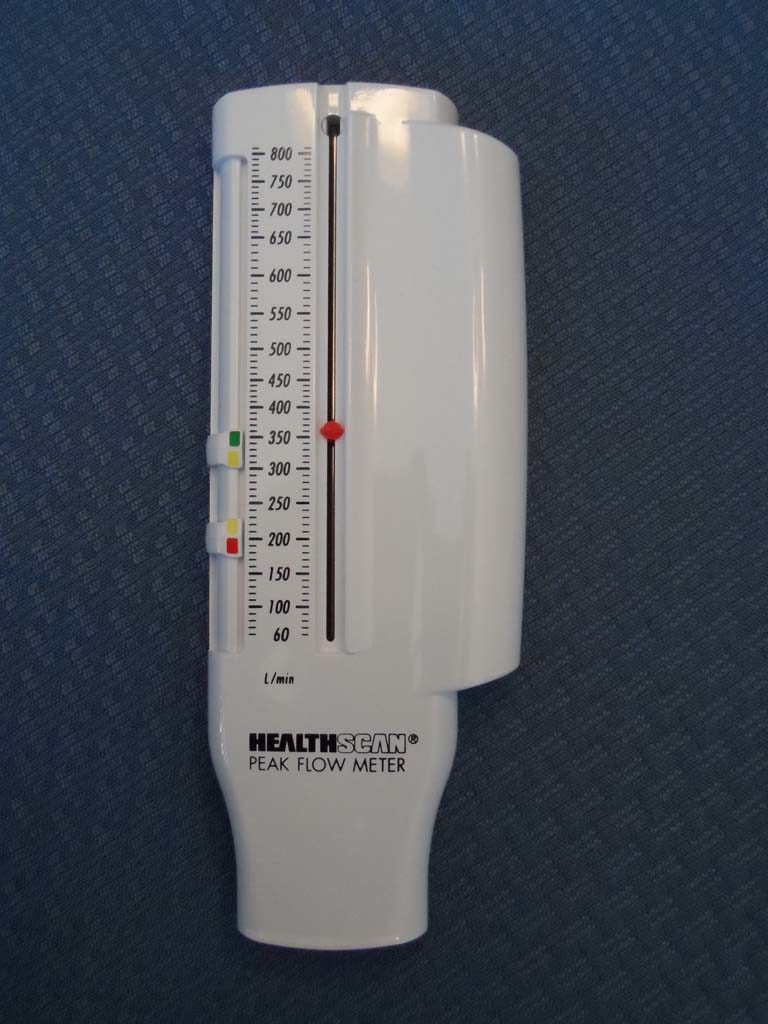
Peak Flow Meter
The patient's green, yellow, and red zones can be set based upon the patient's personal best peak flow. When the peak expiratory flow is measured, the patient can immediately see which zone was reached.
In this picture, the results shown are within the patient's green zone on the Healthscan peak flow meter.
In this picture, the results shown are within the patient's green zone on the Healthscan peak flow meter.
Image from Trudy Watson
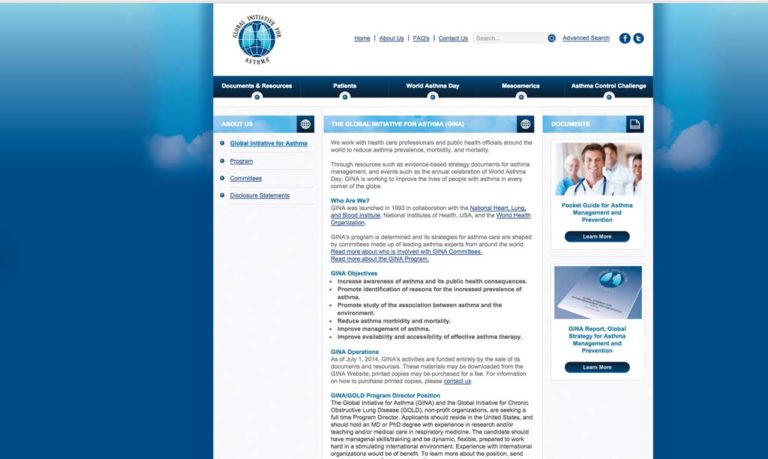
1993 Global Initiative for Asthma (GINA) Established
The Global Initiative for Asthma (GINA) is an international collaborative effort to use evidence-based research to impact the diagnosis, management, and education of asthma. This initiative launched in 1993 with the publication of evidence-based guidelines. The effort was the result of international collaboration between the National Heart, Lung, and Blood Institute and the World Health Organization with the goal of reducing asthma morbidity and morality and improving the management of asthma.
Image from GINA website (www.ginasthma.org)


MDI Reservoirs
A variety of devices are available as reservoirs for aerosolized medication once it is released from the MDI. Many patients have difficulty coordinating their breathing with the activation of the medication release from the MDI. The use of spacers, reservoir bags, and holding chambers helps to increase the amount of medication actually delivered to the patient.


1997 MDI Dose Counter
Auxiliary dose counters for MDIs became available in the 1990s to help patients track the number of doses in their MDI canister.
The Doser is one example of the dose counters and was introduced in 1997.
The Doser is one example of the dose counters and was introduced in 1997.


1997 NAEPP Guidelines
The recommendations for asthma management were developed by the the National Heart, Lung, and Blood Institute's National Asthma Education and Prevention Program (NAEPP) which was formed in 1989. The experts were charged with developing evidence -based clinical practice guidelines for the diagnosis and management of pediatric and adult asthma. The experts included representatives from the AARC. The first guidelines were published in 1991. The second set of guidelines were published in 1997.
Image from Trudy Watson
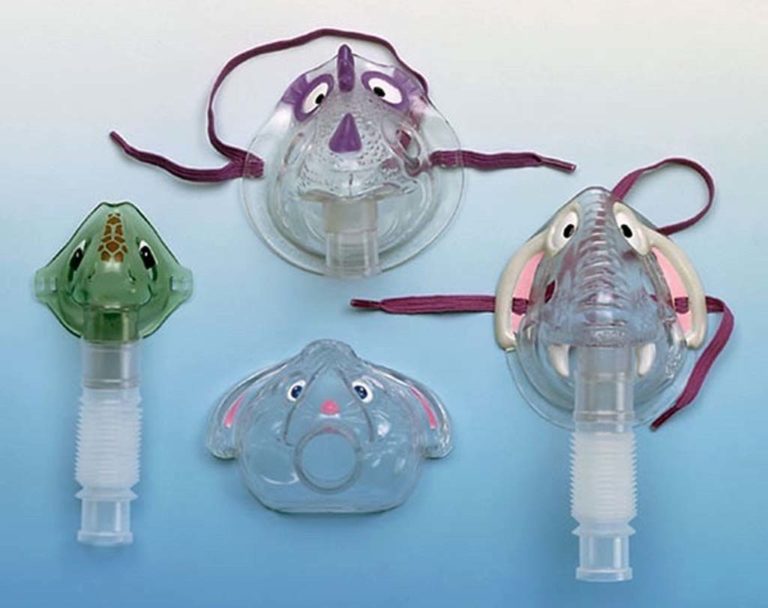

Peds Aerosol Masks
In an effort to enhance compliance with nebulizer treatments among young pediatric patients, a variety of "critter" aerosol masks became available starting in the late 1990s.
These are just a few that are now available.
These are just a few that are now available.


Pediatric Compressors
To enhance pediatric compliance with neb treatments, many companies began to offer compressors with designs that would appeal to children and their parents.
The compressors pictured were among the many options available in 2015.( Medquip markets the five units shown.)
The compressors pictured were among the many options available in 2015.( Medquip markets the five units shown.)


2000s
Asthma treatments from the 2000s are featured.
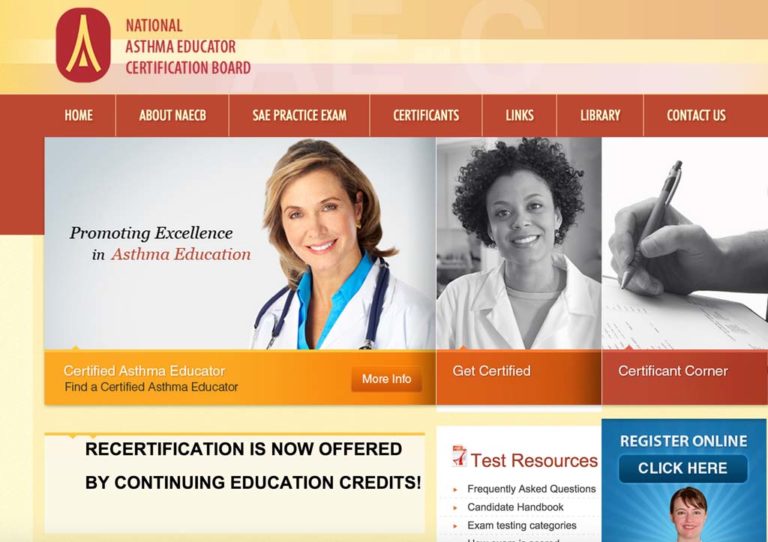
NAECB Established: AE-C Credential Offered
The National Asthma Education Certification Board (NAECB) was formed in 2000 and offered its first asthma educator certification exam in September 2002. Licensed or credentialed health professions who are eligible to take the asthma educator certification exam include respiratory therapists, nurses, nurse practitioners, pulmonary function technologists, pharmacists, physicians, physician assistants, health educators, physical therapists, occupational therapists, and social workers. Individuals who successfully complete the exam are awarded the AE-C credential.
Image from NAECB website (www.naecb.com)


2003 Asthma Guide for RTs
In May 2003, an asthma management guide for respiratory therapists was published by the National Institutes of Health. The publication was the collaborative effort of the AARC, Us Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, and the National Asthma Education and Prevention Program.
Image from Trudy Watson


2003 mAb (omalizumab) Approved
A monoclonal antibody (mAb), Xolair (omalizumab) was approved by the FDA in 2003 for the treatment of difficult persistent cases of asthma in children over the age of 12 years who meet specific criteria. The drug is an IgG monoclonal antibody that requires injection every two to four weeks under direct medical supervision and monitoring. Depending on the dose and frequency of administration, annual costs may range between $10K- $30K.
New mAb therapies continue to emerge, as well as a variety of new agents, new combinations of existing agents, and new delivery devices.
New mAb therapies continue to emerge, as well as a variety of new agents, new combinations of existing agents, and new delivery devices.

2007 EPR-3 Released
The third comprehensive update (EPR-3) of NAEPP's asthma management guidelines was released in 2007.


The Future?
The push continues to reduce the morbidity and mortality associated with asthma. Research is impacting technology and pharmacology. The result of this research has resulted in a multitude of new medications, delivery devices, and therapies for the management of asthma.
For over seven decades, respiratory therapists have fulfilled an essential role on the asthma management team and will continue to do so in the future.
For over seven decades, respiratory therapists have fulfilled an essential role on the asthma management team and will continue to do so in the future.


Images to Share?
Please follow the instructions to contribute photos for this gallery.